WelderDestiny › Welding Engineering Tools › Corrosion Resistance of Stainless Steels
Corrosion Resistance of Stainless Steels
The corrosion resistance of stainless steels can be significantly affected by the welding operation. We therefore would like a way to predict the corrosion resistance, and measure it, if possible. In particular, the pitting resistance of the stainless steels are important. The Pitting Resistance Equivalent Number (PREN) is a way of calculating the pitting resistance of different alloys and welds. The ASTM G48 corrosion test is a more practical way of assessing the pitting resistance of higher alloyed stainless steels.
The WelderDestiny Compass: Weekly e-zine Subscription
You can take a look at "The WelderDestiny Compass" back-issues by clicking here.
Besides
the pitting resistance, we will also look at other factors that can affect the
corrosion resistance of stainless steels when we weld them.
Pitting Resistance Equivalent Number (PREN)
In relation to the corrosion resistance of stainless steels, it
has been noted that Molybdenum tends to increase the pitting resistance. This is why type 316 stainless steel has a better pitting
resistance than type 304 stainless steel. Type 316 contains around 2.5%
Molybdenum whereas type 304 does not contain Molybdenum.
 Heat Tint on Root of Stainless Steel Weld Due to Poor Backing Purge.
Heat Tint on Root of Stainless Steel Weld Due to Poor Backing Purge.Besides
Molybdenum, (Mo) there are other alloying elements that also tend to have this
effect of improving the corrosion resistance of stainless steels by increasing the pitting resistance. The pitting
resistance equivalent number is a way of taking the percentages of the alloying
elements that improve pitting resistance on stainless steels, and expressing it
in a number that can be compared to other alloys for estimating the pitting corrosion resistance of stainless steels.
The
pitting resistance equivalent number (PREN) is calculated using the following
equation:
PREN
= %Cr + 3.3 x %Mo + 16 x %N
From
this equation, it is clear that Nitrogen (N) has a very large impact in
increasing the pitting corrosion resistance of stainless steels. (It is also a very
strong Austenite former, but that is another story for another day!) The
problem is that Nitrogen is difficult to get into a solid solution in high
percentages, and when we melt the material during welding, the Nitrogen will
tend to escape out of the molten metal. For this reason, welding filler metals
will tend to include other alloying elements to compensate for the reduced
Nitrogen content of welds.
So,
let us compare the differences in PREN between typical 304L, 316L and type 2205 Duplex stainless
steel:
- Typical composition of 304L: Cr: 18.5%; Mo: 0%; N: 0% = PREN of 18.5
- Typical composition of 316L: Cr: 17%; Mo: 2.5%; N: 0% = PREN of 25.25
- Typical composition of type 2205 Duplex Stainless Steel: Cr: 22.5% Mo: 3.25 N: 0.16 = PREN of 35.78
From
the pitting resistance equivalent numbers given above, it becomes clear that
316L is more resistant to pitting corrosion than 304L, but that the most pitting
resistant of the three materials being compared is the type 2205 Duplex
Stainless Steel. (DSS)
Please
note that often client specifications, or material specifications may require a
minimum PREN for materials in certain services such as sea water service.
Measuring the Corrosion Resistance of Stainless Steels With an ASTM G48 Corrosion Test
The
PREN discussed above is based purely on the chemical composition of the
material. There are however other factors that affect the pitting corrosion resistance of stainless steels,
such as the condition of the surface oxide layer on the stainless steel, or the
exact form that the alloying elements take within the material, or the presence
of localized areas of alloy segregation or loss. For this reason, some codes
and client specifications require a practical test to be done on materials, and
especially weld test pieces, to prove that they have the desired pitting
resistance. Such a test is described in testing standard ASTM G48.
 ASTM G48 Pitting Corrosion Coupon After Testing: Note pitting on weld. This test failed due to the presence of pits.
ASTM G48 Pitting Corrosion Coupon After Testing: Note pitting on weld. This test failed due to the presence of pits.ASTM
G48 describes 6 different “methods”, which are geared to different types of
alloys and also the type of information required. From a welding perspective,
“method A” is the most widely used test, so we will discuss this method in a
little more detail. Method A is simply called the “Ferric chloride pitting
test.”
In
ASTM G48, Method A, a coupon of the material that is to be tested is placed in
a specific ferric chloride solution and heated to a predetermined temperature
and held for a predetermined time at that temperature. Typical temperatures are
25°C for duplex stainless steels (DSS) and 40°C for super duplex stainless
steels. (SDSS) A typical time for the test is 24 hours, although it can be done
longer.
As
an example, the widely used subsea pipeline code DNV-OS-F101 requires 25 Chrome
duplex stainless steels (SDSS) to be tested at 40°C for 24 hours.
The
acceptance criteria vary, but generally it is a pass / fail type test based on:
- There must be no pitting visible when viewed under low magnification of around 20 times.
- The mass loss of the specimen must be lower than a specified limit. As an example, the DNV-OS-F101 subsea pipeline code gives a maximum mass loss of 4.0 g/m2.
Please
note that some standards or specifications only require the absence of pitting,
without specifying a maximum mass loss.
Also
note that ASTM G48 corrosion testing is generally not a standard requirement of
welding codes such as ASME IX. It is usually done to meet the requirements of a
specific material specification or client standard.
It
is important to note that there are many factors that can potentially affect
the outcome of the ASTM G48 test. Factors such as the heat input during welding
and the shielding gas practices are directly relevant to the welding procedure.
Unfortunately, factors such as how the welded coupon is cleaned after welding,
and how the laboratory prepares the test samples, can also have significant
impacts to the results. In this regard I recommend that the cleaning practices
should be such as to reduce the surface roughness and ensure a sound,
continuous oxide layer on the surface. A typical example is that an overzealous
use of wire brushes will result in significant scratches on the material
surface, which will typically increase the mass loss found during the test. It
is best to use chemical cleaning techniques to remove any unwanted oxides from
the surface and leave a sound oxide layer on the affected areas.
The
ASTM G48 standard is really geared toward the testing of base metals, not
welds. As such it recommends that samples are prepared by “polishing” with 120
grit abrasive paper. This is not really practical for welds that have uneven
and curved surfaces, hence the typical use of alternative surface preparation
methods such as stainless steel wire brushes and chemical cleaning. Such
alternative surface treatments are allowed by the ASTM G48 standard.
Sensitization of Stainless Steels
When
the temperature on a stainless steel increases, the speed at which atoms can
diffuse through the metal’s structure increases. At the same time, the speed of
any chemical bonding / reactions also increase. These effects can lead to
problems with the corrosion resistance of stainless steels when we weld them. One of the problems is sensitization.
In
sensitization, any carbon within the stainless steel will tend to react with
the Chromium to form chrome carbides. As a general rule, the carbon will be
most concentrated within the grain boundaries of the stainless steel. When the
carbon then forms the chrome carbides, a lot of chrome is removed from the
solid solution just next to the grain boundaries. These grain boundary areas
are then depleted of the main alloying element that gives stainless steels
their anti-corrosion properties. A stainless steel that has been affected in
this way is said to be “sensitized”.
 Microstructure of Austenitic Stainless Steel Suffering From Sensitization.
Microstructure of Austenitic Stainless Steel Suffering From Sensitization.When
a sensitized stainless steel is exposed to a corrosive environment, there
will be a preferential corrosive attack along the grain boundaries. This type
of attack can occur very rapidly, and can result in catastrophic failure of the
affected component or structure. In short, sensitization is very bad for the corrosion resistance of stainless steels.
The
temperature at which this sensitization occurs in stainless steels is generally
accepted to be between 500°C and 800°C, although for some alloys it can be as low
as 420°C and as high as 850°C. Below the sensitizing temperature range the
diffusion of the atoms in the material, along with their reactivity is not
enough to result in the sensitization to occur. Above the sensitization temperature, the
chrome carbides tend to dissolve back into the base metal, reversing the negative effect on the corrosion resistance of stainless steels.
It
therefore becomes clear that when welding a stainless steel, there will always
be a band along the weld edges that will be exposed to the sensitizing
temperature range. The longer the material is exposed to the sensitizing
temperature, the greater the amount of sensitization. When a welded component
displays reduced corrosion resistance of stainless steels along the sides of the weld, due to this sensitization, it
is termed “weld decay”.
In
earlier days this reduced corrosion resistance of stainless steels due to sensitization was a significant problem, but it has
been largely eliminated by a number of alloy refinements, such as:
- Low carbon grades of stainless steels: The lower the carbon content of the “L” grades (e.g. 304L and 316L) means that there is less carbon to tie up the chromium in chrome carbides. The low carbon grades have carbon contents of lower than 0.03%. The main down side of the low carbon grades is that their strength at moderately high temperatures are lower than the grades with higher carbon content.
- Stabilized grades of stainless steel: Alloying elements such as Titanium (Ti) and Niobium (Nb) (Also called Columbium (Cb)) form carbides preferentially to chromium. When the stainless steel is alloyed with small quantities of titanium or niobium, then the carbon is tied up by these elements, ensuring that the chrome content is not lowered significantly. This largely solves the sensitization problem. Typical stabilized alloys are type 321, (Ti stabilized) and type 347 (Nb stabilized) stainless steel. Under specific circumstances, stabilized grades can also suffer from chrome depletion leading to very narrow bands of corrosion on either side of the weld. This is termed “knifeline attack”. As a general rule however, this is not a problem, but must be borne in mind.
The corrosion resistance of stainless steels that have been sensitized, can be returned by taking the temperature of the entire component high
enough to return the chromium into solution and then cooling rapidly. Typically
this will be a temperature in excess of 1000°C. Unfortunately, this operation
is often not practical. Once large structures have experienced sensitization,
not much can be done other than replacing the affected material.
There
are standard tests to find out if the corrosion resistance of stainless steels have been reduced due to sensitization. Sometimes
these are required by client standards when higher carbon grades of stainless
steel needs to be able to withstand corrosive environments. One standard to
which such testing is typically done is ASTM A262.
Stress Corrosion Cracking (SCC)
Another
way in which welding can affect the corrosion resistance of stainless steels is
by introducing conditions that can lead to stress corrosion cracking. Stress
corrosion cracking occurs in many different materials, in the presence of
different corrosive media, but it is especially prevalent in austenitic
stainless steels in the presence of chlorides.
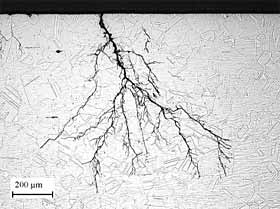 Micrograph of Chloride Stress Corrosion Cracking of Austenitic Stainless Steel.
Micrograph of Chloride Stress Corrosion Cracking of Austenitic Stainless Steel.SCC
occurs when the following three conditions are met simultaneously:
- A susceptible material.
- Exposed to a specific corrosive environment. This would include a specific corrosive, at a suitable temperature and in an electrolytic environment. (In other words, there needs to be a liquid medium that can lead to the flow of ions through the liquid.)
- The material must be experiencing a tensile stress. The higher the stress level, the greater the susceptibility.
In
the classic SCC of austenitic stainless steels, the factors come together as
follows:
- An austenitic stainless steel such as 304 or 316 stainless steel.
- The most common corrosive environment is a solution which contains chlorides. (e.g. sea water) For the SCC to occur, the temperature typically needs to be above 60°C. The higher the temperature the faster the SCC will occur.
- Any weld results in high residual stresses in the weld and the material directly adjacent to the weld.
Due
to this very prevalent SCC mechanism in the presence of chlorides, this
situation has been given its own name. It is called Chloride Stress Corrosion Cracking (CSCC) of austenitic
stainless steels, and is one of the main factors to consider when evaluating the corrosion resistance of stainless steels for any application.
If
the component is small enough, then the SCC corrosion resistance of stainless steels can be restored by relieving the welding related stresses. This can be achieved by subjecting the component to a quench anneal
heat treatment following welding. For many components this treatment is however not practical. This means that it is almost impossible to
prevent CSCC of welded austenitic stainless steels under the operating conditions
mentioned. Under those conditions, other more resistant alloys need to be used.
Typically duplex stainless steels, ferritic stainless steels, super austenitic
stainless steels or nickel or copper based alloys.
Reduced Corrosion Resistance of Stainless Steels Due to Surface Contamination
The
corrosion resistance of stainless steels is reliant on a surface layer of a
very adherent chrome oxide. This chrome oxide layer forms all by itself in the
presence of oxygen. When the surface layer is damaged, it generally tends to
re-form rather rapidly in the presence of oxygen, restoring the corrosion
resistance. The big secret is that there needs to be oxygen present to re-form
the chrome oxide layer. Under conditions where the material is starved of
oxygen, rapid localized corrosion is possible.
 Corrosion Due to Surface Contamination of Pipe Spools: Note that these are new spools, so the corrosion has been very rapid.
Corrosion Due to Surface Contamination of Pipe Spools: Note that these are new spools, so the corrosion has been very rapid.Such
oxygen starved conditions are typically found locally on the surface of stainless steels under the following
circumstances:
- In crevices in the stainless steel. (Leading to crevice corrosion.)
- Coatings that are not very adherent to the surface of the stainless steel. Once again, introducing a crevice like condition.
- Thick oxide layers that are not as adherent as the thinner oxide layers. This can happen when stainless steels are heated to high temperatures in the presence of oxygen. This often happens in the heat affected zones of stainless steel welds where the shielding has not been adequate. Again, any breakdown of this thicker oxide layer will result in a very localized crevice, leading to pitting corrosion. An additional issue is that the thick oxide layer was formed at the expense of some of the chrome close to the stainless steel’s surface, making these surface areas depleted in chrome and therefore less resistant to the corrosive attack.
- Where material of lower corrosion resistance has perforated the surface oxide layer. Typically this happens when ordinary iron or steel gets embedded in the surface oxide layer. In this case there is a galvanic corrosion effect on the iron, causing it to rust away in that local spot, essentially introducing a small localized crevice. At that point, pitting corrosion will typically start.
It
is therefore very clear that any operations that result in “heat tinting” of
the material, such as welding, can severely reduce the corrosion resistance. In
addition, any operation that can introduce iron contamination into the surface
oxide layer can also reduce the corrosion resistance. This reduction of the corrosion resistance of stainless steels can easily happen if
tools contaminated with iron is used on the stainless steels.
Typical
examples of this are:
- Using grinding discs on stainless steels that were previously used on steel.
- Letting grinding sparks from steel impinge on stainless steels.
- Handling stainless steel with steel slings.
- Using ordinary steel wire brushes on stainless steel.
- Rolling stainless steel on steel rollers, or using any steel tools on stainless steel.
The
best approach is to try to eliminate any source of surface contamination of the stainless
steel. Where some contamination or heat tinting has occurred, or is inevitable
(e.g. you do not have the necessary stainless steel tooling) then performing a
pickling and passivation treatment on the stainless steel’s surface can restore
the corrosion resistance of stainless steels.
WelderDestiny › Welding Engineering Tools › Corrosion Resistance of Stainless Steels
The WelderDestiny Compass: Weekly e-zine Subscription
You can take a look at "The WelderDestiny Compass" back-issues by clicking here.